-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
eurine-act
-
-
-
-
-
-
-
-
-
european network for the study of adrenal tumors

eurine act
EURINE-ACT was an international prospective study that examines the diagnostic and prognostic value of urine steroidobolomics in patients with adrenocortical tumours. Urine steroidobolomics is the combination of steroid profiling by gas chromatography/mass spectrometry (GC/MS) followed by data analysis by machine learning analysis. The study is led by Professor Wiebke Arlt from the University of Birmingham.
In preceding cross-sectional studies the Birmingham has established through collaboration with ENS@T that urine steroidobolomics can differentiate between benign and malignant adrenocortical tumours by a simple urine test that appears to have a higher specificity and comparable sensitivity as imaging techniques.
This will now be tested prospectively in the EURINE-ACT trial with many centres from ENS@T and the UK Adrenocortical Tumour Network UK-ACT already actively recruiting.
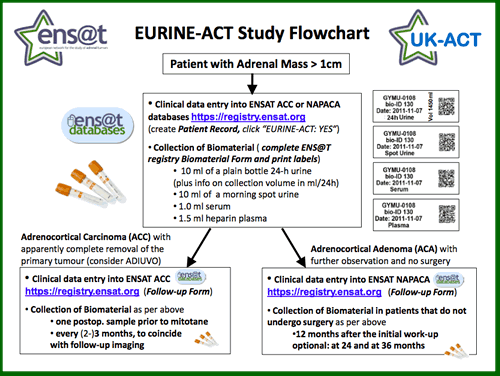
The scheme below gives a brief overview of the study (click the image below to download the full PDF). We aim to recruit patients with any adrenal mass >1cm and require entry of the clinical information into the appropriate ENS@T database (ENS@T ACC for adrenocortical carcinoma, ENS@T NAPACA for any other adrenal mass/adrenal incidentaloma). The required biomaterial consists fo a 24-h urine, a spot urine, a serum and a heparin plasma sample.
Patients with confirmed ACC can participate in the EURINE-ACT ACC follow-up arm that offers urine steroidobolomics every 3 months following apparent complete surgical removal of the primary tumour. This arm aims to assess the sensitivity of the approach for detection of recurrence in comparison to imaging and will also analyse steroid changes induced by mitotane treatment.
Patients with an adrenal incidentaloma that is thought to be an endocrine inactive adrenocortical adenoma can participate in the EURINE-ACT ACA follow-up arm, with biomaterial required 6 and 12 months after initial diagnosis and then annually. This arm aims to detect whether the adrenal incidentalomas show evidence of potentially deleterious excess hormone production during the first three years after initial diagnosis.
THIS PROJECT IS ALREADY CLOSED